![SOLVED: (a) basic buffer solution with pH 10.5 is prepared by dissolving NHACl into 30 mL 0.15 M ammonia, ammonium chloride, NH; solution. [Given: Kb for NH; 1.8 X 10-] Calculate the SOLVED: (a) basic buffer solution with pH 10.5 is prepared by dissolving NHACl into 30 mL 0.15 M ammonia, ammonium chloride, NH; solution. [Given: Kb for NH; 1.8 X 10-] Calculate the](https://cdn.numerade.com/ask_images/5b0b09243de64cee9ee5f6101c584fab.jpg)
SOLVED: (a) basic buffer solution with pH 10.5 is prepared by dissolving NHACl into 30 mL 0.15 M ammonia, ammonium chloride, NH; solution. [Given: Kb for NH; 1.8 X 10-] Calculate the

How can we calculate the pH of the solution in which 0.2 M NH4Cl and 0.1 M NH3 are present and the pKb of ammonia solution is 4.75 .

pH of when 50mL of 0.10 M ammonia solution is treated with 50 mL of 0.05 M HCI solution :- ` - YouTube

In the titration of 50.0 mL of 0.10 M ammonia (K_b = 1.8 times 10^{-5}), calculate the pH: 1 ) Before titration begins 2 ) After addition of 20.0 mL of 0.10 M hydrochloric acid 3 ) After addition | Homework.Study.com
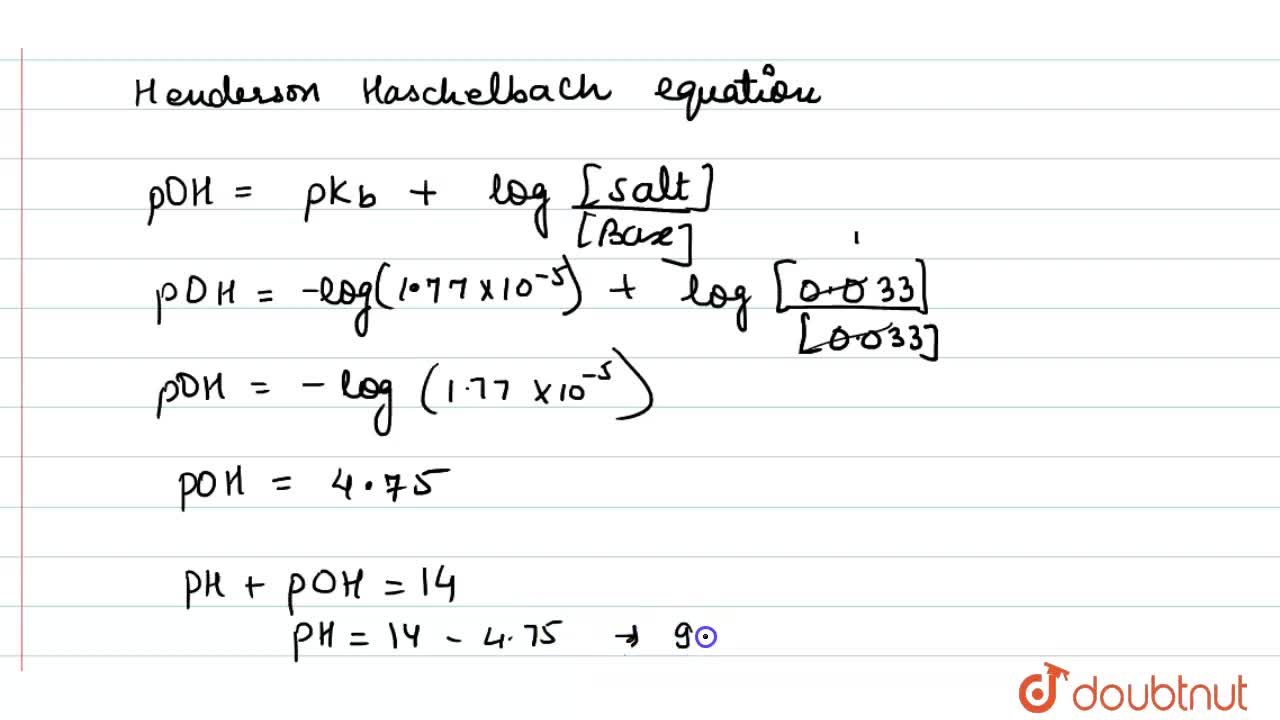
Calculate the pH of 0.033 M ammonia solution if 0.033 M NH(4)Cl is introduced in this solution at the same temperature (K(b) for NH(3)=1.77xx10^(-5))

Calculate the pH of 0.10M ammonia solution. Calcualte the pH after 50.0mL of this solution is treated with 25.0mL of 0.10M HCl. The dissociation constant of ammonia, K(b)=1.77xx10^(-5).

SOLVED:The pH of a solution of household ammonia, a 0.950 M solution of NH3, is 11.612 . Determine Kb for NH3 from these data.